Latest information
一年以内の更新履歴はありません。
Japanese CDMO and related information materials
This document is a compilation of non-confidential slide deck from companies belonging to FIRM CDMO sub-committee. If you have inquiries regarding specific companies, please refer to their respective websites or contact FIRM at info@firm@or.jp.
Management Policy/Role
Mission
For sound market formation of medical care with regenerative medicine (RM) under “The Act on the Safety of Regenerative Medicine (ASRM)”, the Committee contribute through discussions to establish ecosystem of Specific Processed Cells Manufacturers (Registered/Permitted/Certified Facilities), to solve the challenges at medical institutions that provide medical care with RM and also to share the information of RM with patients in respect of R&D activities and medical cares for patients of RM with using Specific Processed Cells.
Strategic Direction
The committee will try to identify the challenges, to which Specific Processed Cells Manufacturers are facing, to improve the business environment through the discussions with its stakeholders such as Japanese Society for Regenerative Medicine (JSRM), other academic societies for therapeutics of various diseases, MHLW, PMDA and etc., and to make lobbying activities for sound business environment.
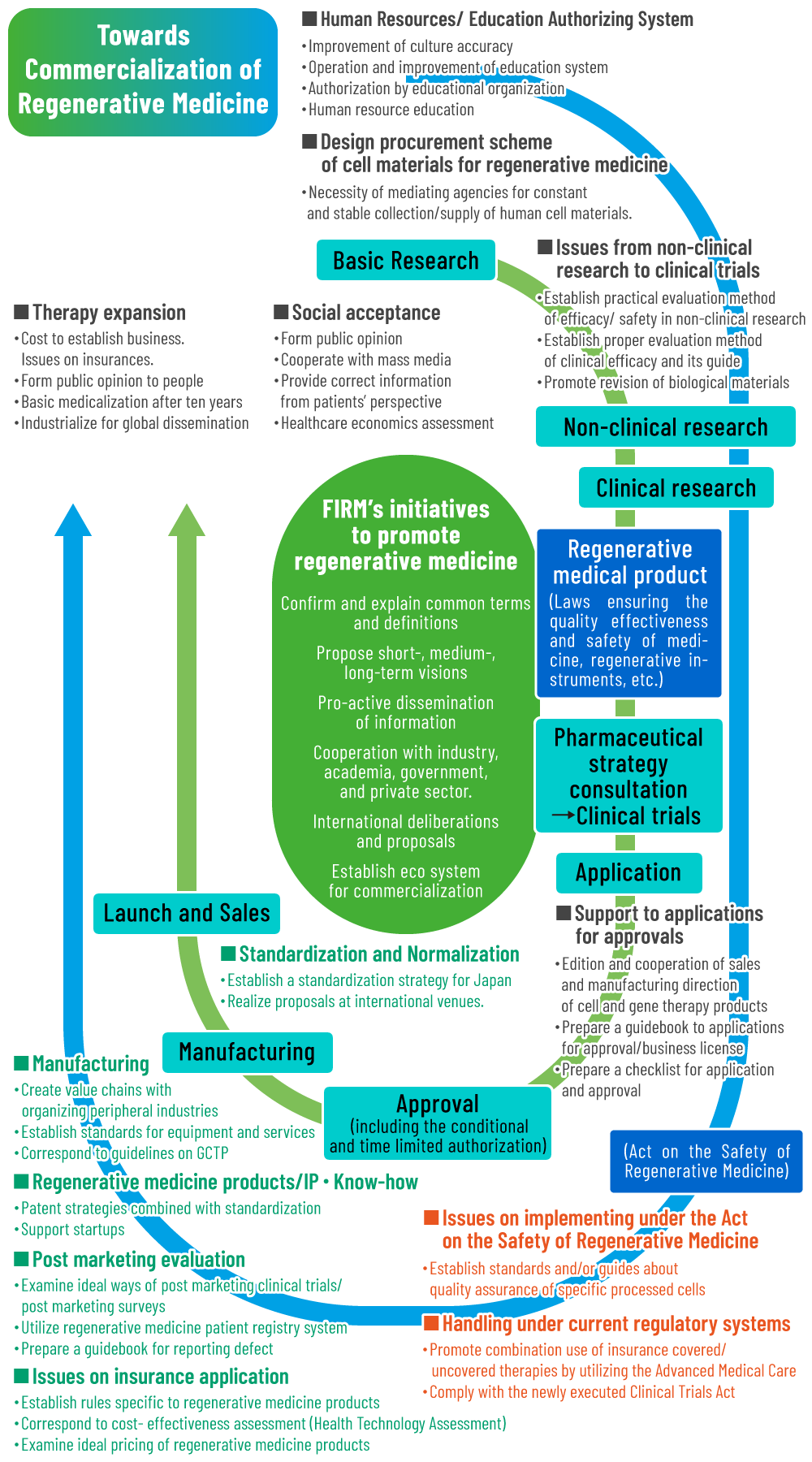
Challenges on practices of ASRM
- Establishment of Standard/Guide on QA/QC/QMS for Specific Processed Cells
Regulatory Issues
- Promotion of healthcare services combining insurance covered and non-covered services by Advanced Medical Treatment
- Regulatory harmonization with Clinical Research Act
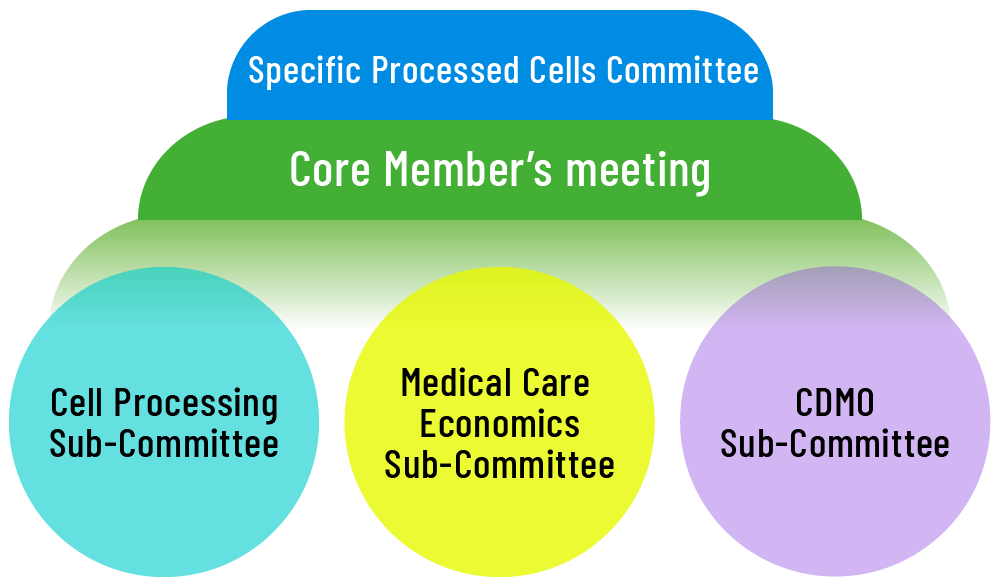
Sub-committees, targeted to solve the identified challenges
- Cell Processing
Sub-Committee - Medical Care Economics
Sub-Committee - CDMO
Sub-Committee
Organization
Action Plan FY2022
1. Differences on Capabilities among 3 categories of Specific Processed Cell Manufacturers (Registered/Permitted/Certified Facilities) and also differences within each category will be well observed, and the opinion on countermeasures to such differences will be summarized. In addition, the exchanges of opinions about such countermeasures will be coordinated with JSRM and relevant administrative authorities of the Government.
2. Activities such as internal seminars for understanding the background of “The System on Healthcare Services combining Insurance Covered and Non-Insurance Covered Services (Combined System)” by Advanced Medical Treatment and the National Healthcare Insurance System (especially reimbursement and pricing mechanisms) will be arranged. The activities to exchange opinions and to make consensus regarding how to apply to Combined System for Medical cares under ASRM, including such discussion with JSRM will be started. In addition, the activities to understand the private healthcare insurance systems, which could be utilized to reduce financial burdens for patients will be started.
3. The actions to clarify the conditions to utilize clinical data from Clinical Research works and Data from actual clinical treatments, and to understand Patient Clinical Data Registry systems will be started.
4. The market survey over market trends and future technology trends will be made based upon its necessity in order to recognize the current situation of such trends. Then, targeted technologies and products/services will be specified, and challenges and its solutions will be identified, including the collaboration with JSRM and relevant administrative authorities of the Government.
5. The internal seminars to learn how to apply to be listed as Insurance Covered medical cares under National Healthcare Insurance System will be held.
6. The supportive activities for the steps on Accreditation over Products/Services provided by FIRM Supporting Industries Committee members will be coordinated.
Action Plan Cell Processing Sub-Committee
Manufacturing of Specified Processed Cells under ASRM such as hardware, software and its practices should be standardized among Registered/Permitted/Certified Facilities and its Qualities should have equal footing.
Action Plan Medical Care Economics Sub-Committee
As challenge to accelerate medical cares with RM under ASRM, apart from Manufacturing, “The System on Healthcare Services combining Insurance Covered and Non-Insurance Covered Services (Combined System)” – out of scope from Prohibition of Combined Treatment should be introduced and provide benefits for patients.
Purpose of activities of the CDMO Sub-Committee
Coming soon.
