Latest information
Purpose (Mission)
Contribute to industrialization of and promotion of expansion of regenerative medicine through maintenance and activation of value chains of peripheral industries around regenerative medicine.
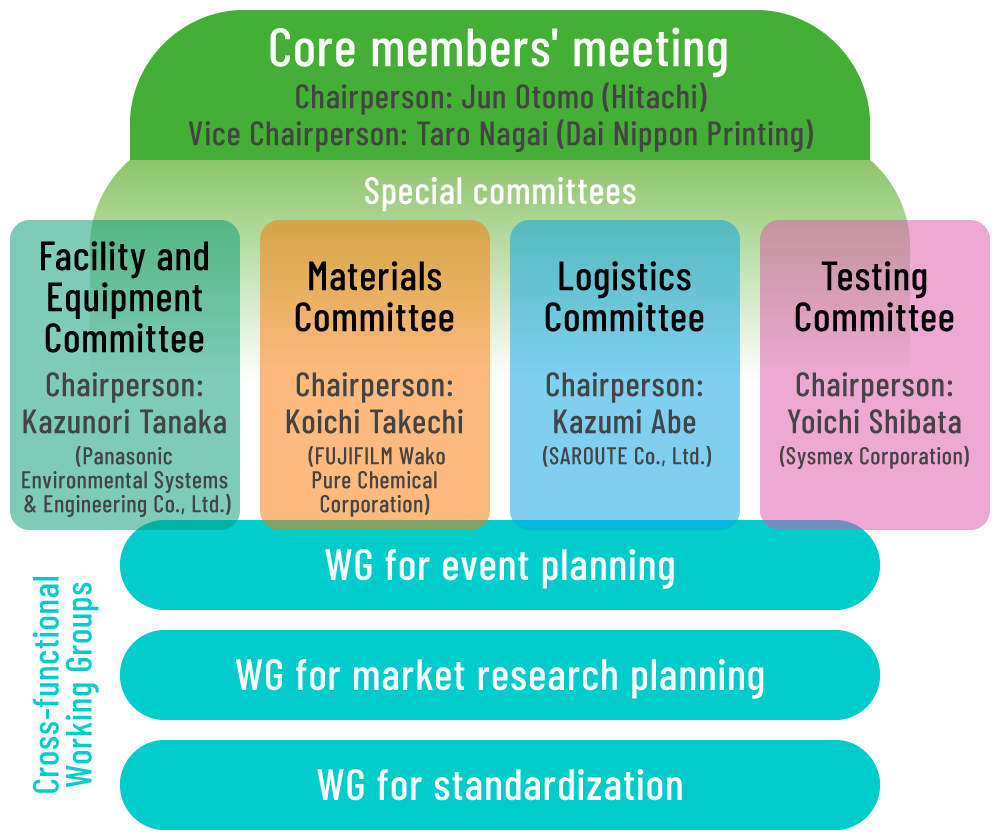
Organization
Action Plan
1. Organization and main activities
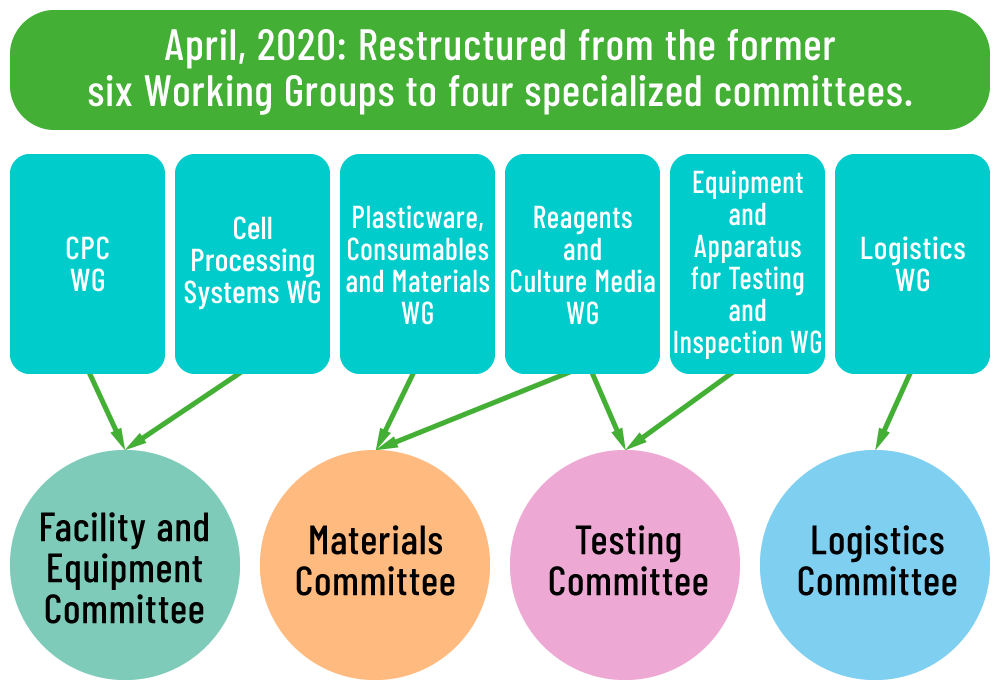
1. Discuss problems on each product and service and promote activities such as study groups in these four specialized committees.
- Facility and Equipment Committee
- Handle facilities, equipment and among others.
- Materials Committees
- handle culture, material cell, equipment and among others.
- Testing Committees
- Handle equipment and reagent for testing.
- Logistics Committees
- Handle logistics, storage, biobank, and others.
2. Set up the below cross-functional WGs to handle common themes among the specialized committees.
- WG for event planning
- Handle planning and secretariat of each event.
- WG for market research planning
- Plan and practice market research and actual condition survey about peripheral products and services.
- WG for standardization
- Promote system for realization of FIRM standard and support for standardization of the exist FIRM standard, working together with the Standardization Committee.
3. Supervise activities and decide policy in the Core members’ meeting consisted from chairpersons and vice chairpersons from the committees.
2. Cooperation with Cell and Gene Therapy Products Committee
Provide discussion with Manufacturing committee of Cell and Gene Therapy Products Committee to extract and organize issue in manufacturing, testing, and logistics, and to deepen mutual understanding. Moreover, discuss solutions and gather them into FIRM standard to announce.
3.Cooperation with ministries and cabinet offices, and academia
Exchange opinions regularly with METI, MHLW, PMDA and the Japanese Society for Regenerative Medicine. Convey issues and requests from peripheral companies and require support to committee activities such as FIRM standardization.
4. Action plan of each committee
Facility and Equipment committee
- Extract issues of existing facilities and equipment
- Consider ideal state of the low-risk facility.
- Exchange information and opinion with regulatory authorities
- Plan and implement equipment study meetings.
Material committee
- Extract and suggest issues of materials.
- Investigate biological raw materials standards.
- Hold related study meetings.
- Edit user manual on culture and reagent
- Produce Q&As for equipment user manual.
Testing committee
- Extract issues of testing.
- Hold related study meetings.
- Follow up case study for testing equipment
(Approach to the low risk facilities which offer regenerative medicine.)
Logistics committee
- Extract issues of logistics.
- Collect information on regularity and market in Japan and abroad.
- Consider needs for standardization in logistics field.
- Plan and implement all kinds of event, e.g. facility tour.
- Study the translation of ISO 21973:2020(international standard of transporting cells for therapeutic use), and the review of FIRM standard which has already produced and disclosed.
Performance
October 16, 2020: Exhibited and presented at Regenerative Medicine JAPAN 2020
Exhibited at FIRM booth in Regenerative Medicine JAPAN 2020, from Oct 14 to 16, and introduced activities at Regenerative Medicine Stage.
https://www.ics-expo.jp/saisei/ja/
April 1, 2020: Restructured organization
Restructured Supporting industries committee. Accelerate activation of peripheral industries.