Title: Development of International Standards for supporing industries of regenerative medicine and establishment of certification system for them
Summary
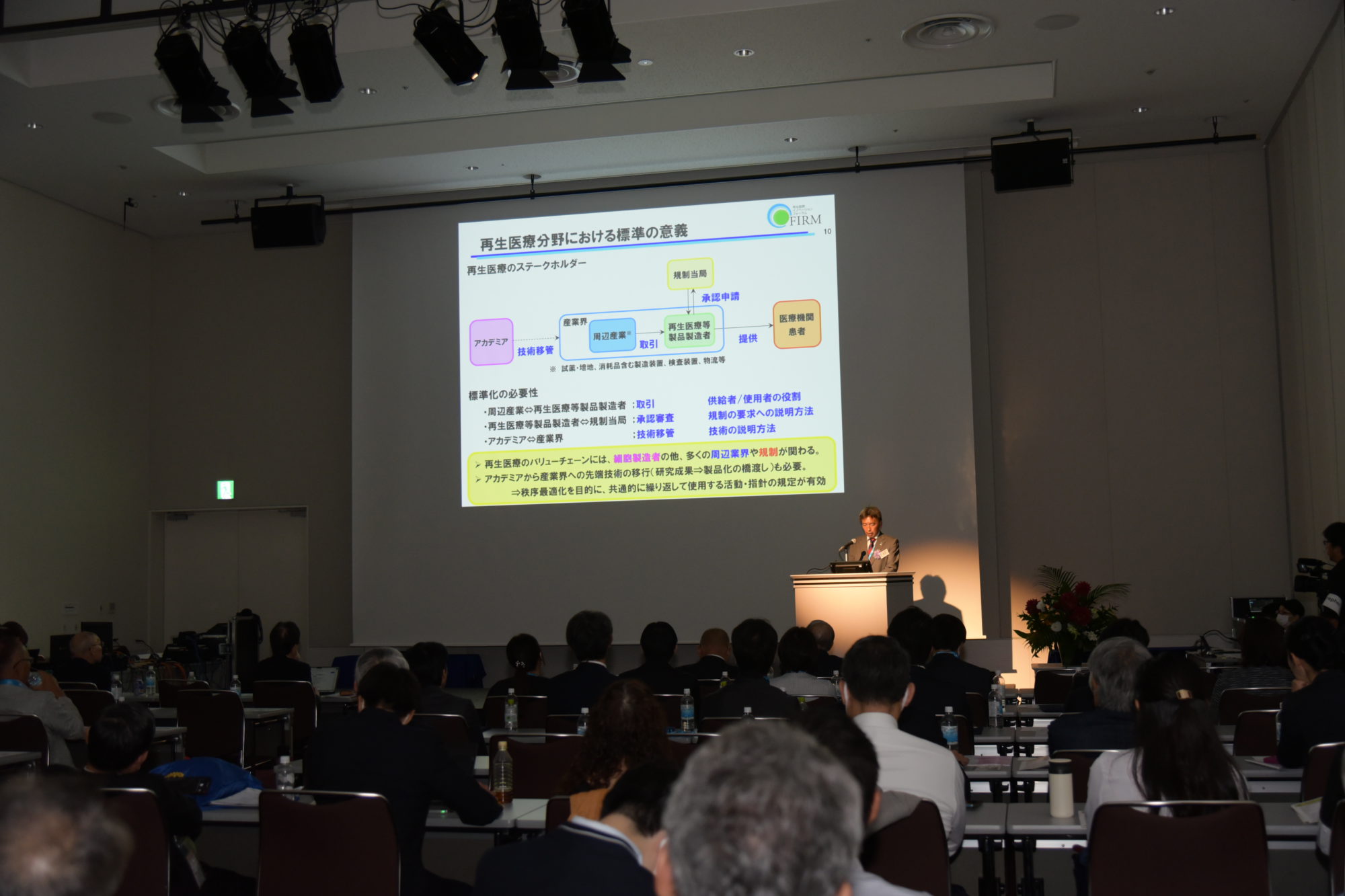
In ISO/TC276/WG4(Biotechnology- Bioprocessing for cells and related entities), we led the development of international standards relating ancillary material, manufacturing equipment including consumable, packaging, and transportation, which are the products and services of supporting industry for regenerative medicine, to clearly define the requirements for both suppliers and users. Furthermore, we have established a product certification system based on above standards by establishing certification scheme owner based on ISO/IEC 17067 (Confomity assessment – Fundamentals of product certification and guidelines for product certification schemes) and a certification body that conforming to ISO/IEC 17065 (Confomity assessment – Requirements for bodies certifying products, processes and services) at the Forum for Innovative Regenerative Medicine.
Japan is the world’s first country to have established the certification system for the supportng industry for regenerative medicine. As this system spreads, it is expected to secure consistency in the process from basic research to manufacturing of regenerative medicine, thereby promoting the industrialization of regenerative medicine as well as the export of Japanese products and services.
-
Awarding lecture in Bio Japan 2024 -

Awarding celemony
