Participation committee
The following committees participated
Booth exhibit report
FIRM exhibited at BioJapan/Regenerative Medicine JAPAN2022 held on-line with Pacifico Yokohama on October 12-14 this year as well. We would like to express our sincere gratitude for the many visitors. Panels of Supporting Industries Committee, Cell and Gene Therapy Products Committee, and Specific Processed Cells Committee were displayed in the FIRM booth. These three committees and the Public Relations Committee assigned briefings there.
-

Panel: Specific Processed Cells Committee -

Panel: Supporting Industries Committee -
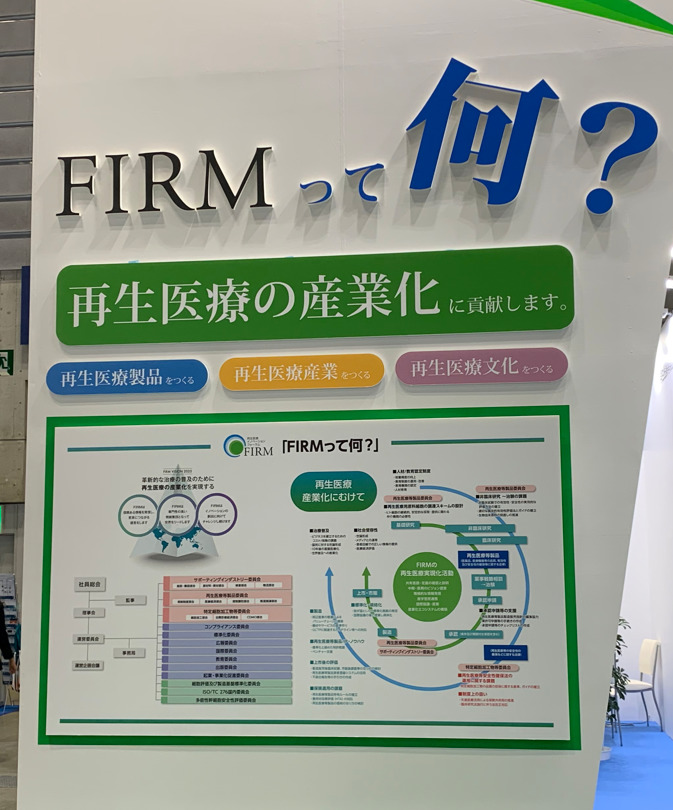
Panel: Introduction to FIRM
Report: Organizer's seminar
October 12

Title: Challenges and actual conditions for ecosystems in regenerative medicine – focusing on the manufacturing of regenerative medicine
Experts were invited to discuss how the manufacturing ecosystem for regenerative medicine should be aimed by Japan.
October 13
-

Speaker: FIRM Representative Director President Hata -

Panelist: FIRM Director Kimura
Title: Towards universalization of regenerative medicine (cell therapy, gene therapy)-Challenges to diversity
In order to universalize regenerative medicine, we introduced the government’s ideas on research and human resource development, the possibility of using data, including post-marketing, and the introduction of a system adapted to the diverse nature, and conducted discussions between experts from various fields and product companies.
October 14
-

Coordinator: FIRM Vice President Director Hirose -

Venue from the behind
Title: Latest trends in regenerative medicine and other products
As of September 2022, 17 regenerative medicine products have been approved. we presented the recently approved regenerative medicine and other products, and how they will be marketed and developed in the future.
Open Stage Seminar
-

Venue -

Presentation by Supporting Industries Committee
-

Presentation by Specific Processed Cells Committee -

Presentation by Venture Establishment / Promotion Committee
